Fetal And Neonatal Monitoring Helps In Constantly Monitoring Vital Parameters Of The Fetus During Labor And Delivery
Fetal and neonatal monitoring is a critical tool used to monitor fetal health during labor and delivery. It helps to determine whether a baby is growing and developing normally, and can detect problems that require immediate attention such as low fetal oxygen saturation or premature birth.
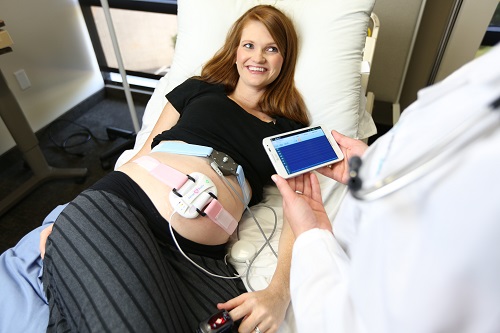
The most common form of fetal monitoring is external
continuous fetal monitoring (EFM). This requires the provider to place two
measuring devices around the abdomen to monitor uterine contractions and fetal
heart rate. These devices include a pressure transducer to measure uterine
contractions and an ultrasound device to measure fetal heart rate.
Most
healthcare providers prefer to use Fetal
and Neonatal Monitoring Market
as it is quick and easy, especially when compared to
intermittent auscultation. It has limitations, as it may not detect abnormal
fetal heart rate patterns that may indicate a fetal distress problem, and it
has high false positive rates and inconsistent fetal heart rate tracing
interpretations.
Some providers may also choose to use a more invasive
internal fetal monitoring method. This involves inserting a catheter into the
uterus to monitor contractions. It can be uncomfortable, whereas it may help
when there is trouble getting an accurate reading with external methods.
Some
hospitals provide continuous electronic fetal monitoring devices that are
portable. Fetal and Neonatal Monitoring Market are waterproof, can be taken in a
shower, and have wireless capabilities. They are useful for women who need to
move around or labor in a bed-less position away from the hospital bed.
Fetal and Neonatal Monitoring Market are becoming more popular as they
allow the provider to track fetal and maternal progress during labor without
having to move the baby. They may also be more comfortable to use and are often
more affordable than traditional fetal monitors.
Many obstetrical professionals like EFM as it gives them proof
that the baby is doing fine during labor. It is also a good way to keep records
of the baby's condition, which can help in defending medical malpractice
lawsuits.
However, more obstetric professionals are concerned about
the risks associated with EFM and are reluctant to use it in their practice.
These professionals may have been influenced by the increased number of studies
that find that EFM carries risks and doesn't lead to better newborn outcomes.
Nuvo
Group gained approval from the United States Food and Drug Administration in
June 2021, for expanding their INVU. A new uterine activity module can be added
to their prescription-initiated remote pregnancy monitoring platform that will
enable remote monitoring of the uterine activity.
Comments
Post a Comment