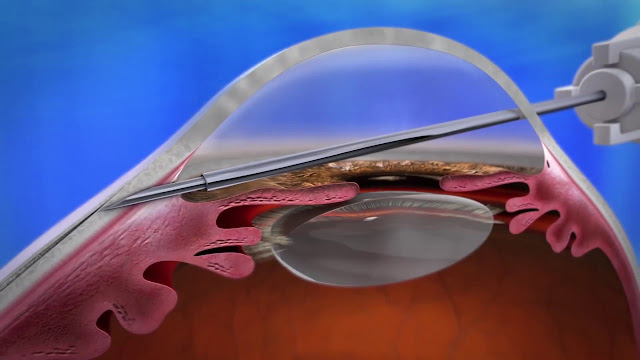
A stent is a tiny, inflatable tube used to treat arteries that are too narrow. It is used to strengthen weak arteries and prevent them from breaking. For the creation of gel stents, soft gelatin is employed. The aqueous humor in the sub-conjunctival space is drained by the injection of a gel stent, which becomes hydrated and decreases intraocular pressure. It's a minimally invasive process.
The
adoption of new technologies by hospitals and clinics is one of the primary
factors propelling the global Gel
Stent Market.
The most recent technological developments in Gel stents have increased demand
worldwide. The market is comprehensively evaluated in the study on the global Gel Stent Market.
A Stent is a tiny, inflatable tube used to treat arteries that are too narrow. Additionally, it can be inserted into weak arteries to enhance blood flow and stop arteries from bursting. Stents are particularly useful in keeping the arteries from bursting and in supplying blood to the weak arteries. Soft collagen that is produced from gelatin makes up the Gel Stent. The Gel Stent Market is made using gelatin that is soft and produced from collagen. After being injected, Gel Stent becomes hydrated and swells, creating a soft, flexible tube that clings to ocular tissue and is immobile. It's a minimally invasive process.
In comparison to other
synthetic materials, it greatly lowers intraocular pressure (IOP) by allowing
fluid to flow into the subconjunctival tube and decreases complications.
Clinical trials are divided into preclinical, clinical phase I/II, clinical
phase III, research, clinical phase I, and research clinical phase II, depending
on the clinical trial. The first micro-invasive glaucoma surgery device, the
iStent is the smallest stent that can be grafted into the body (MIGS device). Gel Stent is extensively used in eye research facilities. Hospitals and eye
clinics also employ them medically.
Key Players
In order to create
technologically cutting-edge items and broaden their product line, key players
are concentrating on research and development. Early in 2017, the XEN
(Allergan) was introduced in the United States after receiving FDA approval in
November 2016. Allergan, Alcon, Infocus, and Glaukos Corp. are significant
players in the global gel stent market.
Comments
Post a Comment